Download PDF A quantum chemical dialogue mediated by textbooks Pauling's The nature of the
savvychemist Intermolecular Forces (2) Permanent Dipole Bonding
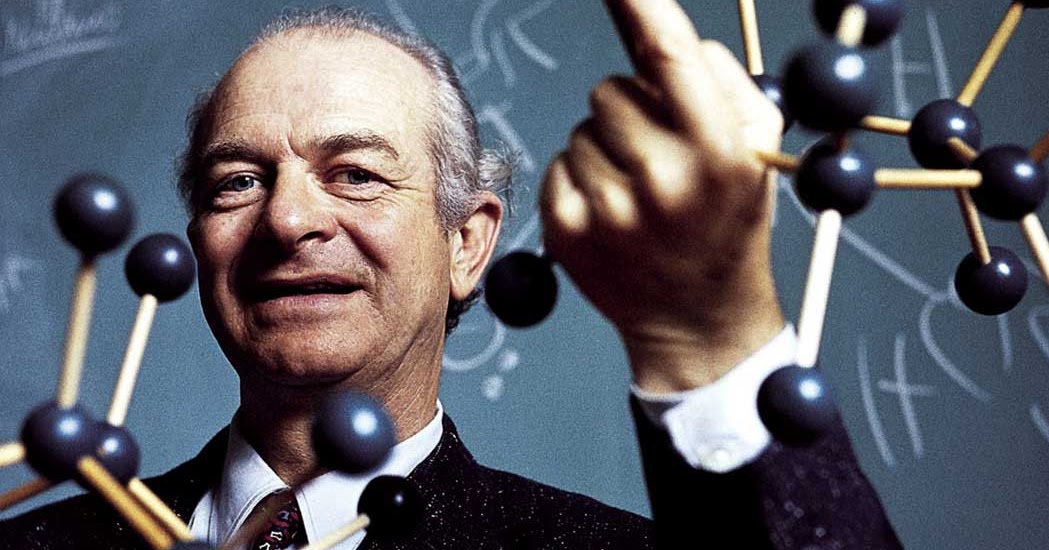
The nature of the chemical bond and the structure of molecules and crystals : an introduction to modern structural chemistry. Pauling, Linus, 1901-1994. Publication date 1960 Topics Chemical bonds, Quantum chemistry, Molecules, Crystallography, Chemistry, Molecular Structure, Crystallography, Ligacoes Moleculares, Chemische binding.
The Nature of the Chemical Bond by Linus Pauling Etsy

In 1926 Linus Pauling, then a promising young doctoral candidate, set sail for Europe to study quantum mechanics with an eye toward applying this new physics to problems in structural chemistry.. awarded for "research into the nature of the chemical bond and its application to the elucidation of complex substances." Start by reading our.
Berkeley Lectures. March April 1931. Page 11 (Large Version) Correspondence Linus Pauling

Linus Pauling's The Nature of the Chemical Bond has, like Isaac Newton's Principia or Charles Darwin's On the Origin of Species, the kind of iconic status that, for some, removes any obligation to.
The Nature of Chemical Bond

THE NATURE OF THE CHEMICAL BOND. APPLICATION OF RESULTS OBTAINED FROM THE QUANTUM MECHANICS AND FROM A THEORY OF PARAMAGNETIC SUSCEPTIBILITY TO THE STRUCTURE OF MOLECULES. Linus. Pauling; Cite this: J. Am. Chem. Soc. 1931, 53, 4, 1367-1400. Publication Date (Print): April 1, 1931. Publication History. Published online 1 May 2002; Published.
LINUS PAULING NATURE Chemical Bond 1960 3. Auflage Cornell Univ HC VG++ NM Nobelpreis EUR 78,42

Nature of the Chemical Bond. Second edition, revised (Pauling, Linus) Sidney J. French ; Cite this: J. Chem. Educ. 1940,. The Role of d Orbitals and Electron‐Rich Multi‐Center Bonding. Angewandte Chemie 2015, 127 (41) ,. American Chemical Society About. About ACS Publications; ACS & Open Access; ACS Membership;
Lecture Notes Quantum Mechanics. 1927 1928. Page 29 (Large Version) Correspondence Linus

The pre-publication announcement of the third edition of The Nature of the Chemical Bond resulted in the largest advance sale of any technical book in the history of Cornell University Press.
Advertisement for The Nature of the Chemical Bond. 1939. Pictures and Illustrations Linus

The Nature of the Chemical Bond and the Structure of Molecules and Crystals. An Introduction to Modern Structural Chemistry. By Prof. Linus Pauling.
The Nature of the Chemical Bond Linus Pauling Quantum Mechanics Chemistry

The Nature of the Chemical Bond. By Linus Pauling. Cornell University Press, Ithaca, N. Y. 450 pages, $4.50. - Volume 8 Issue 1
THE NATURE OF THE CHEMICAL BOND. APPLICATION OF RESULTS OBTAINED FROM THE QUANTUM MECHANICS AND
"Statement by Linus Pauling about the Manuscript on the Nature of the Chemical Bond." August 6, 1979. "There was this long gap from 1928 when I wrote my first paper on quantum mechanics of the chemical bond in the Proceedings of the Royal Society, and 1931 when I wrote the first significant paper.Well, there was this gap because I was having so much trouble getting a result that was in simple.
Linus Pauling, American Biochemist Photograph by Science Source Fine Art America

The Nature of the Chemical Bond provides a general treatment, essentially nonmathematical, of present (as of 1960) knowledge about the structure of molecules and crystals and the nature of the chemical bond. Among the new features in the third edition are a detailed resonating-valence-bond theory of electron-deficient substances, such as the boranes and ferrocene; a chemical theory of the.
301 Moved Permanently

by. Linus Pauling. This book is a seminal work in the field of chemistry, providing an in-depth exploration of the concept of the chemical bond, including its properties and the principles governing its behavior. It synthesizes quantum mechanics with chemical phenomena, offering a comprehensive framework for understanding molecular structure.
The Nature of the Chemical Bond 9780801403330 Linus Pauling Boeken

Pauling received the Nobel Prize in Chemistry in 1954 "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances." One of Linus Pauling's many peacekeeping activities—flipping pancakes at the 1963 pancake breakfast of the Pasadena branch of the Women's.
Linus Pauling lecturing amidst several molecular models. 1960s. (Large Version) Pictures and

PAULING'S CHEMICAL BOND The Nature of the Chemical Bond and the Structure of Molecules and Crystals An Introductfon to Modern Structural Chemistry. By Prof. Linus Pauling. Third edition.
diagram Linus pauling, Chemical bond, Diagram

The Nature of the Chemical Bond provides a general treatment, essentially nonmathematical, of present (as of 1960) knowledge about the structure of molecules and crystals and the nature of the chemical bond.. Among the new features in the third edition are a detailed resonating-valence-bond theory of electron-deficient substances, such as the boranes and ferrocene; a chemical theory of the.
Linus Pauling (19011994)., ‘The Nature of the Chemical Bond. Application of Results Obtained
.jpg?mode=max)
The pre-publication announcement of the third edition of The Nature of the Chemical Bond resulted in the largest advance sale of any technical book in the history of Cornell University Press.. Pauling, Linus. The Nature of the Chemical Bond: An Introduction to Modern Structural Chemistry, Cornell University Press, 1960. Pauling, L. (1960).
Electronegativity Bond Scale Surfguppy Chemistry made easy for visual learners
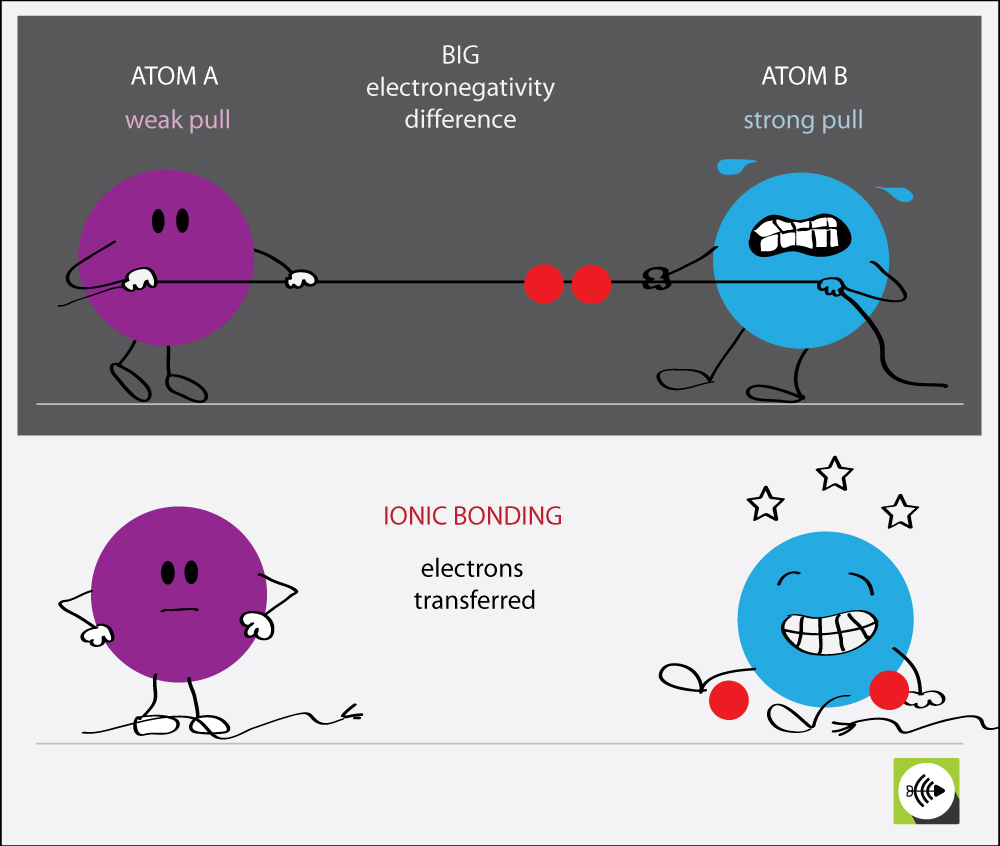
Pauling L. The nature of the chemical bond Cornell Univ. (1960) is a classic book on the theory and application of chemical bonding, written by the Nobel laureate Linus Pauling. It covers topics such as molecular structure, resonance, hybridization, electronegativity, and crystal chemistry. The book is available in PDF format on Academia.edu, a platform for sharing and accessing academic research.
.